Просмотров: 5 433
Dermatomyositis/Polymyositis
Irina Alexandrovna Zborovskaya – doctor of medical sciences, professor,cathedral professor of hospital therapy with the course of clinical rheumatology of the doctors improvement faculty of Volgograd state medical university, director of the Federal Budgetary State Institution (FBSI) “Research and development institute of clinical and experimental rheumatology” of the RAMS, head of the regional Osteoporosis Center, presidium member of the Association of rheumatologists of Russia, member of the editorial boards of the magazines “Scientific and practical rheumatology” and “Modern rheumatology”
Definition. Dermatomyositis (DM) is a disease characterised by systemic involvement of striated muscles and characteristic dermatologic manifestations. It belongs to the group of systemic diseases of the connective tissue. If skin is not involved, the term “polymyositis” (PM) is typically used.
Epidemiology. DM can occur in people of any age. The incidence of dermatomyositis has been estimated 5 cases per million people every year. DM affects women more frequently than men (3:1 ratio); DM with an associated malignancy affects men as frequently as women (1:1 ratio); DM with characteristic connective tissue findings affects women ten times as frequently as men (10:1 ratio); inclusion body myositis (IBM) affects men 3 times as frequently as women. Two peak ages of onset exist. In adults, the peak age of onset is approximately 35 – 60 years, and, in children, the peak age is approximately 11 – 17 years. Polymyositis in adults is often associated with a malignancy. Polymyositis with an associated malignancy amounts to 20 – 30% of all cases. It usually affects adults older than 50.
Aetiology and pathogenesis. Aetiology remains unknown. Dermatomyositis and polymyositis as well as some other diseases are often referred to as “idiopathic inflammatory myopathies” as they are usually associated with characteristic muscular findings.
Viral aetiology and the effect of some infectious agents should be also considered. Some cases of dermatomyositis and polymyositis after rubella, herpes infection, administration of vaccines and sera as well as some drugs have been reported. Exposure to cold, insolation, increased physical activity have been suggested as possible triggers of the disease. High-titred antibodies to picornaviruses are typically revealed in blood of children with DM. High – titred antibodies to Toxoplasma gondii species are found in blood of adults with PM. However, the attempts to obtain viruses from the muscles of patients and to reproduce myositis in experimental animals by means of administering the extracts of muscular tissue of the sick were unsuccessful. Thus, the role of infectious agents, including viruses in pathogenesis of DM is uncertain.
Nevertheless, we should consider that the formation of autoantibodies to cytoplasmic proteins is associated with immune reactions to changed viruses whose antigens are the same as those of the body.
A genetic predisposition may also exist. Cases of DM in first-degree relatives have been reported. It may be linked to certain human leukocyte antigen HLA types (for example, B8, DR3, DRW52).
In most patients with inflammatory myopathies autoantibodies to nuclear antigens (ANA) may be present. Circulating immune complexes form in tissues, muscles, skin and vessels resulting in immune complex inflammation. The development of DM associated with a malignancy may be caused either by a direct toxic effect of tumorous agents on the muscles or by the development of autoimmune reactions due to the link between tumour-specific and muscular tissue antigens.
There is evidence for the role of cellular immune disturbances in the development of DM and PM. It has been suggested that muscles are involved in PM due to increased T-cell cytokine toxicity. These cytokines induce aberrant expression of major histocompatibility complex (MHC) class I molecules on muscle cells. Destruction of muscle fibres and their infiltration by sensitized lymphocytes and macrophages usually occur. PM is caused by the direct effect of lymophotoxins produced by lymphocytes on muscle fibers (perforin and granzim).
Pathomorphology. Striated musculature is most commonly affected. Myocardium and skin are involved less commonly. Lymphocytes and plasmatic cells usually prevail in inflammatory infiltrates. Macrophages, eosinophils and neutrophils may be also present. In PM CD8+ cells infiltrate muscle fibers, while in DM CD4+ cells and B-lymphocytes accumulate in the area of small vessels.
Muscle involvement is manifested by focal necrosis, destruction of muscle fibers and loss of transverse striation.
 Signs of regeneration of skeletal muscles are manifested by increased amounts of muscular nuclei as well as basophilia of muscle fibers. In chronic DM interstitial fibrous infiltrates often occur.
Signs of regeneration of skeletal muscles are manifested by increased amounts of muscular nuclei as well as basophilia of muscle fibers. In chronic DM interstitial fibrous infiltrates often occur.
 In a prolonged course of DM muscular atrophy resulting in the formation of dense connective tissue is often observed. Irreversible fibrous contractures typically develop.
Skin involvement is manifested by atrophy of papillae, thinning-out of the epidermis, thickening and structural changes of collagen fibers.
Classification:
In a prolonged course of DM muscular atrophy resulting in the formation of dense connective tissue is often observed. Irreversible fibrous contractures typically develop.
Skin involvement is manifested by atrophy of papillae, thinning-out of the epidermis, thickening and structural changes of collagen fibers.
Classification:
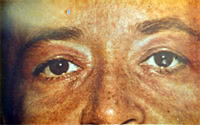 Erythema in DM is quite stable and is often associated with edema, which is sometimes similar to Quincke’s edema, especially at an initial stage of the disease.
Erythema in DM is quite stable and is often associated with edema, which is sometimes similar to Quincke’s edema, especially at an initial stage of the disease.
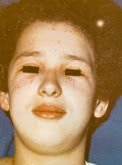 Soft tissues are typically enlarged, pasty-like when palpated, covered with pruritic erythema; less commonly they are associated with hemorrhages, telangiectasias, ulcers and hyperkeratosis. Pathognomonic changes occurring in DM include heliotrope (“sun”) eye-lids (that is stained violet and swollen eye-lids) and Gottron papules consisting of slightly elevated violaceous papules and plaques over finger joints.
Soft tissues are typically enlarged, pasty-like when palpated, covered with pruritic erythema; less commonly they are associated with hemorrhages, telangiectasias, ulcers and hyperkeratosis. Pathognomonic changes occurring in DM include heliotrope (“sun”) eye-lids (that is stained violet and swollen eye-lids) and Gottron papules consisting of slightly elevated violaceous papules and plaques over finger joints.
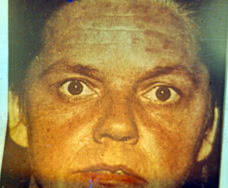 Other symptoms include “mechanic’s hands”, that is hyperkeratotic eruptions over the fingers and palms and “shawl sign”, that is a V-shaped erythema over the anterior neck and upper chest and back. In the course of DM erythema and edema spread over other parts of the body. Atrophy, pigmentation and dryness of skin usually aggaravate.
Other symptoms include “mechanic’s hands”, that is hyperkeratotic eruptions over the fingers and palms and “shawl sign”, that is a V-shaped erythema over the anterior neck and upper chest and back. In the course of DM erythema and edema spread over other parts of the body. Atrophy, pigmentation and dryness of skin usually aggaravate.
 Foci of skin depigmentation may occur. Nail trophic changes and hair loss may be observed. Mucous membranes may be also involved leading to conjunctivitis, stomatitis, edema of fauces and true vocal cords.
Sometimes Raynaud’s syndrome may be present. It usually has a two-phase course and doesn’t cause trophic ulcers. Although cases of periungual bed infarction, petechia and livedo reticularis have been reported.
Muscular syndrome is the key syndrome in diagnosis of DM/PM. Severe, sometimes necrotic myositis of proximal muscles of the extremities is typical of DM. Muscle weakness is usually slowly progressive.
Foci of skin depigmentation may occur. Nail trophic changes and hair loss may be observed. Mucous membranes may be also involved leading to conjunctivitis, stomatitis, edema of fauces and true vocal cords.
Sometimes Raynaud’s syndrome may be present. It usually has a two-phase course and doesn’t cause trophic ulcers. Although cases of periungual bed infarction, petechia and livedo reticularis have been reported.
Muscular syndrome is the key syndrome in diagnosis of DM/PM. Severe, sometimes necrotic myositis of proximal muscles of the extremities is typical of DM. Muscle weakness is usually slowly progressive.
 However, in some cases the disease may have an acute course. In this case it is characterised by a rapid development of acute respiratory and renal (myoglobinuria) failure. Muscle weakness, especially muscles of the pelvic girdle and the thigh is considered to be the primary manifestation of DM. However, the earliest symptoms may include dysphagia and weakness of neck muscles. Symmetric proximal muscle weakness of the extremities is typical. Patients often begin to note weakness of muscles when combing their hair, dressing themselves, climbing stairs, walking, rising from a sitting or lying position, though the movements of hands, fingers and feet may be quite normal.
Larynx and pharynx muscle involvement typically results in dysphagia, nasality (nasal intonation), sometimes aphonia, hoarseness, choking on swallowing. Aspiration of food into the trachea is also possible. Patients with PM/DM have difficulties in swallowing solid and liquid food which sometimes passes through the nose. In DM/PM the upper portion of the esophagus, muscles of the soft palate and the tongue are usually involved.
In case of ocular muscle involvement diplopia and ptosis may develop. When facial muscles are involved, the person may develop a mask-like face. Sphincter involvement typically results in sphincter incompetence, that is involuntary defecation and urination. Intercostal muscle and diaphragm involvement may result in disturbed respiration.
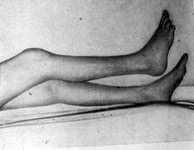
Muscles are usually tender on palpation, swollen, pasty-like, however, they become sclerosed in the course of the disease. Calcium deposits are often seen (that is ossifying myositis). They are typically revealed at X-ray examination of the muscles of the shoulder and pelvic girdles. Calcinosis in PM/DM is usually secondary and is often localized under the skin in the form of plaques or massive deposits in the areas of the shoulder and pelvic girdles where muscles are mostly affected. Areas of fascia and subcutaneous fat which are adjacent to the involved muscles are typically calcified.
Joint syndrome is less typical and is usually manifested by arthralgias or periarticular tissue involvement. Arthitis is rare; however, it is usually symmetric and non-destructive. Disorders of joint functions and the formation of contractures are typically associated with muscle involvement.
Internal organ involvement is seen in most patients with PM/DM but do not prevail in the clinical picture of the disease.
Pulmonary involvement is manifested by muscle weakness which usually spreads over the respiratory muscles, including diaphragm. It may be the cause of reduced respiratory function. Clinical manifestations include accelerated respiration and hypopnoe, inspiratory dyspnea, hypostatic pneumonia, marked pain syndrome. Pulmonary involvement may occur in the form of typical vascular or interstitial pneumonia (for example, pneumonitis, alveolitis) and aspiration pneumonia which is aggravated by respiratory muscle involvement. Pulmonary fibrosis with the signs of pulmonary hypertension and respiratory failure (for example, fibrosing alveolitis) may also occur. It is characterised by increased inspiratory dispnea, dry cough, crackling rales in the lower part of the lungs, pulmonary failure.
Cardiac involvement: myocardium is most commonaly affected; pericardium and endocardium – less commonly. Focal or diffuse myocarditis with dystrophic changes of the myocardium, cardiosclerosis, cardiofibrosis or arrhythmias may develop. Cases of dilated cardiomyopathy have been reported. Constrictive pericarditis is often seen in increased disease activity.
Renal involvement in DM/PM occurs quite rare and varies from focal glomerolonephritis or transient proteinuria to severe diffuse glomerolonephritis and myoglobinuria kidney with features of renal failure.
Gastrointestinal involvement is seen quite often and is manifested by increased dysphagia (due to involvement of the muscles of the fauces and the upper portion of the esophagus), loss of appetite, sometimes pain in the abdomen and symptoms of gastroenterocolitis. Dysphagia typically develops due to a reduction of contractile activity of pharyngeal muscles and muscles of the upper portion of the esophagus, disturbed peristalsis, muscle weakness of the soft palate and the tongue. Sometimes dysphagia may be the earliest symptom of the disease. Esophageal sphincter involvement typically results in reflux esophagitis. Severe form of progressive dysphagia when solid food is regurgitated and liquid food passes through the nose usually poses a threat to the patient’s life.
Neurological disturbances are not commonly seen and are manifested by various changes of sensititvity such as hyperaesthesia of peripheral or radicular type, hyperalgesia, paresthesia or areflexia. Sometimes pseudoneurological symptoms may occur. Poorly marked polyneuritis or even CNS involvement may develop due to vasculitis. Absence of reflexes in the involved extremities results from severe muscle involvement. Electromyography is commonly used to identify the cause of muscular changes.
Endocrine disturbances are quite rare.
Weight loss is considered to be the most common general symptom. Sometimes it may be rather significant. Fever is typically seen in an acute course of the disease or after an exacerbation of the disease. In most patients subfebrile temperature is typically seen.
Lab findings and other tests. Disease activity in DM/PM is characterised by mild anemia, leukocytosis, less commonly by leucopenia, eosinophilia, increased erythrocyte sedimentation rate (ESR), increased concentration of alfa-2 and gamma-globulin, seromucoid, ceruloplasmin, C-reactive protein.
Typical features of PM are elevated levels of muscle enzymes which are found in muscular tissue. They include creatine phosphokinase (CPK), fructose diphosphate aldolase, lactate dehydrogenase (LDG), aspartate and alanine aminotransferase.
Creatine phosphokinase levels may yield abnormal results. For example, its concentration may be 80 times as high as the normal one. Its concentration increases by 5 – 10 times in DM. However, some cases when patients don’t develop increased levels of creatine phosphokinase have been reported. In most patients elevated levels of serum myoglobin are typically revealed. Myoglobinuria is seen less commonly. Enzymeemia activity correlates with the extent of the disease activity and it may be helpful in the assessment of efficacy of treatment.
The range of autoantibodies revealed in patients depends on the type of PM/DM and the presence of concomitant diseases. In most (approximately 80 – 90%) patients with DM/PM antibodies to nuclear or cytolplasmic antigens (for example, Hep-2 cells) are typically present. Positive antinuclear antibodies with ring-like staining are common in patients with inflammatory myopathies.
Inflammatory myopathies induce formation of autoantibodies to myocytes. These antibodies are rarely revealed in other autoimmune diseases and are considered as myositis-specific. They may be divided into 4 groups. They are the following:
1. Antibodies to synthase of transfer RNA (anti-aminoacyl-tRNA-synthase). They take an active part in affection of skeletal muscles (antisynthase antibodies). They interact with cytolplasmic enzymes which mediate the link between aminoacids and transfer RNA. Antibodies to histidil-t-RNA-synthase are more frequent in patients with PM. In PM, especially in patients with interstitial pulmonary involvement, production of antisynthase antibodies is associated with the development of antisynthase syndrome.
2. Antibodies to signal recognition particles interact with one of the components of the cytoplasmic protein complex which is responsible for the transfer of polypeptides through the membranes of endoplasmic reticulum and block it.
3. Anti-Mi-2 antibodies interact with nucleoproteins whose function remains unknown. They do not combine with cytolplasmic proteins of myosin-specific antigens.
4. Antibodies to elongation factor i-alfa which provides transfer of aminoacyl-t-RNA-synthase to ribosomes and its movement along the polysome.
Sometimes muscle biopsy, either of the deltoid muscle or quadriceps, may enhance the clinician’s ability to diagnose DM. The disease which is revealed with the help of skin and muscle biopsy is not specific. It should be considered in diagnostics and differential diagnostics of the disease only in combination with clinical and laboratory findings of PM/DM.
Electromyography (EMG) enables the doctor to reveal reduced amplitude and shortened bioelectric potential of the affected muscles, polyphasy, sometimes – pathologic spontaneous activity such as fibrillation, pseudomyotonic disturbances, etc. EMG findings are not typical of DM/PM; they may vary in the course of the disease. Thus, they don’t enable the clinician to differentiate PM/DM from other myopathies. However, they are widely used in combination with other clinical findings and methods of examinatio
Diagnosis. No international criteria which can aid in the diagnosis and classification of DM/DM exist. However, in 1975 A. Bohan and J.B. Peter suggested a set of diagnostic criteria and in 1985 Т. Меdsger and А. Маsi suggested a set of classification criteria which are used in the diagnosis and classification of DM/DM. Seven diagnostic criteria of DM/PM are usually singled out. They are the following:
1) Common skin changes
2) Progressive proximal symmetrical muscle weakness (according to the patient’s past history and physical examination)
3) Elevated levels of one or more serum muscle enzymes
4) Abnormal (myopathic) findings on electromyogram (EMG)
5) Abnormal findings (polymyositis) on muscle biopsy
6) Increased creatinuria
7) Reduction of muscle weakness after corticosteroid therapy.
According to A Bohan and J.B. Peter, who suggested first five criteria, the presence of the first criterion or any other three criteria enables the doctor to make the diagnosis of DM. When the first criterion or any other two criteria are present, DM is considered to be likely. When the first criterion or some other criterion is present, DM is considered to be possible.
In PM 4 out of 5 criteria enables the clinician to make the diagnosis of certain PM. When 3 out of 4 criteria are present, PM is considered to be likely. When 2 out of 4 criteria are present, PM is considered to be possible.
These criteria were revised by Tahimoto et al. in 1995. They include the following:
1. Skin changes:
a) Heliotrope skin rash (light-violet erythema with edema of the upper eye-lids);
b) Gottron papules;
c) Erythema on the back surface of the joints of the extremities It is slightly raised. A slight scale may be present. Light-violet erythema in the knee and elbow joints is also common;
2. Proximal muscle weakness (upper or lower extremities and the trunk);
3. Elevated levels of creatine phosphokinase (CPK) or aldolase;
4. Pain in muscles when they are pressed or spontaneous pain;
5. Electromyogramm changes such as short polyphase bioelectric potential, fibrillation and pseudomyothic discharge);
6. Revealing of antisynthase antibodies;
7. Non-destructive arthritis or arthralgias;
8. Features of systemic inflammation (body temperature is usually higher than 37 C°, increased C-reactive protein or erythrocyte sedimentation rate levels – more than 20 mm/hour are present).
9. Myositis revealed in biopsy material (that is infiltration of skeletal muscles with inflammatory cells and focal or extensive degeneration of muscle fibers leading to necrosis and regenerative processes with irregular substitution of muscle fibers for fibrosis).
If any cutaneous manifestation and at least 4 other criteria are present, DM is highly possible. If 4 criteria from the second to the ninth are present, the diagnosis of PM is probable.
Differential diagnostics. Despite typical clinical manifestations of PM, its diagnostics, especially at an initial stage, may be somewhat difficult. PM should be differentiated from other diseases, which are associated with proximal muscle weakness and marked myalgias. PM should be differentiated from:
1. Infectious myositis
1) Viral myositis (coxsackieviruses, ECHO, influenza and HIV viruses)
2) Myositis in toxoplasmosis
3) Myositis in trichinosis
4) Myositis in cysticercosis
2. Metabolic myopathies
1) Acute alcohol myopathy
2) Mc Ardl’s disease
3) Carnitine-palmitil-transferase deficiency
4) Glicogenosis Type V
5) AMP-desaminase deficiency
3. Endocrine myopathies
1) Hypothyroidism
2) Hyperparathyroidism (primary and secondary)
3) Osteomalacia of any aetiology
4. Hypokalemia (prolonged administration of diuretics), administration of statins, hemofibrozil, epsilon-aminocapron acid and other drugs.
A number of diseases which should be differentiated from DM/PM are characterised by proximal muscle weakness and are not associated with myalgias. They are the following:
1. Demyelinating plyneuropathies
2. Intermittent porphyria
3. Myasthenia
4. Lamber-Iton’s syndrome
5. Spinal amyotrophy of Kugelberg-Welander
6. Progressive muscular dystrophies:
a) Extremity-girdle Erb’s disease
b) Facial-shoulder-scapular (myodystrophia Landuzi-Dejerine).
7. Hypercorticism, hyperthyroidism, acromegalia
8. Acid maltase deficiency
9. Chronic alcohol myopathy
10. Guillain-Barre’s syndrome
11. Drug-induced myopathies (caused by penicillamin, azothimidin, cimetidin, colchicines, glucocorticoids, cyclosporine, azathioprin, etc.)
It is important not only to diagnose PM/DM, but also to determine its clinical form and to perform differential diagnostics of primary (idiopathic) and secondary (tumorous) DM/PM.
PM/DM is often associated with rheumatic diseases. These diseases amount to 20% of all overlap syndromes. DM/PM is most commonly associated another connective- tissue disease or systemic scleroderma, rheumatoid arthritis or systemic lupus erythematosus. Less commonly DM/PM are associated with nodular periaatheriitis or rheumatism. It is usually diagnosed if clinical and laboratory findings of myositis and one or more connective-tissue diseases are present.
Treatment. Large doses of glucocorticoids (GC) help control muscle involvement in most patients. The minimum effective dose of GC is about 1mg/kg for children and adults. The earlier the treatment is started, the more effective it will be. Large doses of GC should be administered for 2 – 4 months until clearly marked signs of improvement appear. These signs usually include reduction of muscle weakness and facial edema, improvement of phonation, swallowing, appearance of pigmentation in the area of erythema as well as improvement of laboratory findings and muscle enzyme activity.
However, creatine phosphokinase activity doesn’t help decide on efficacy of treatment. Glucocorticoids in the dose of 1 mg/kg should be administered for 4 months before one decides on efficacy of treatment. A more prolonged period (not less than 6 months) is necessary for regeneration of muscle fibers. It should be considered when monitoring the patients and estimating efficacy of treatment.
There are different treatment and dosage options; however, the treatment of each patient should be strictly individualized. The doctor should evaluate the patient’s condition, efficacy of treatment, tolerance of the selected drug, etc. The maintenance dose should be selected and it should be administered for some years; however, in stable clinical remission the maintenance dose can be reduced or even withdrawn.
In increased clinical and laboratory disease activity, presence of visceral organ involvement and progressive myopathy pulse therapy with high doses of methylprednisolone (1000 mg) is typically administered. Methylprednisolone is usually administered intravenously with 0, 9% isotonic solution of sodium chloride (500 ml) or 5% glucose solution. The course of therapy lasts for 3 days.
Cytostatic drugs are also used in the treatment of PM/DM. Most experts believe that cytostatics are not effective without adequate glucocorticoid therapy. When cytostatic drugs are administered in combination with glucocorticoids, they have a “steroid-saving” action. It means that they give the possibility to achieve clinical effect with low doses of glucocorticoids). In DM/PM metotrexat, azathioprin, chlorbutin and cyclophosphamid are used .
Indications for administration of cytostatics are the following:
1. PM/DM resistant to maximum doses of glucocorticoids.
2. Presence of concomitant diseases or adverse effects, which limit the administration of glucocorticoids.
3. Unresponsiveness of some patients to glucocorticoid therapy.
Methotrexate which has few side effects and is characterised by low carcinogenicity compared with other cytotoxic drugs is the most beneficial.
Azathyoprin ranks the second, whereas alkylating agents such as Cyclophosphan, Chlorbutin should be indicated only if methotrexate and azathyoprin therapy was ineffective.
Oral methotrexate should be given in the dose of 7.5 mg to 25 – 30 mg weekly. Treatment should be started with a small dose. The dose should be gradually increased by 0.25 mg weekly until the optimum one. In patients who can`t take methotrexate orally, it should be given should be intravenously. Intravenous methotrexate should be started with 0.2 mg/kg once a week, increasing the dose by 0.2 mg/kg every 7 days.
Toxicity is evaluated after 6 days following the beginning of treatment and when the cumulative dose of 1500 mg is reached, liver biopsy is usually indicated. The dose of methotrexate should be reduced gradually, considering clinical and laboratory findings and creatine phosphokinase levels.
Azathyoprin isadministered in the dose of 2 – 3 mg/kg. Medical dose is 100 – 150 mg daily. The maximum clinical effect is typically revealed only 6 – 9 months after the beginning of treatment.
The maintainance dose is 50 mg daily. It should be decreased by 25 mg according to the same pattern as with methotrexate. According to Steinberg (1991), if methotrexate and azathyoprine are ineffective, combined treatment with 2 drugs should be undertaken.
Cyclophosphamide is rarely effective, but it is typically selected in interstitial pulmonary fibrosis. In PM/DM it should be used in patients who are unresponsive to methotrexate and azathyoprine. Cases of effective treatment with Chlorbutin (4 mg daily) in patients who received high doses of glucocorticoids, methotrexate and pulse therapy with methylprednisolone without marked clinical effect have been reported. There is evidence for successful use of Cyclosporine A in patients with juvenile DM and in adults with DM, especially in interstitial pulmonary fibrosis.
However, in some cases the disease may have an acute course. In this case it is characterised by a rapid development of acute respiratory and renal (myoglobinuria) failure. Muscle weakness, especially muscles of the pelvic girdle and the thigh is considered to be the primary manifestation of DM. However, the earliest symptoms may include dysphagia and weakness of neck muscles. Symmetric proximal muscle weakness of the extremities is typical. Patients often begin to note weakness of muscles when combing their hair, dressing themselves, climbing stairs, walking, rising from a sitting or lying position, though the movements of hands, fingers and feet may be quite normal.
Larynx and pharynx muscle involvement typically results in dysphagia, nasality (nasal intonation), sometimes aphonia, hoarseness, choking on swallowing. Aspiration of food into the trachea is also possible. Patients with PM/DM have difficulties in swallowing solid and liquid food which sometimes passes through the nose. In DM/PM the upper portion of the esophagus, muscles of the soft palate and the tongue are usually involved.
In case of ocular muscle involvement diplopia and ptosis may develop. When facial muscles are involved, the person may develop a mask-like face. Sphincter involvement typically results in sphincter incompetence, that is involuntary defecation and urination. Intercostal muscle and diaphragm involvement may result in disturbed respiration.
Muscles are usually tender on palpation, swollen, pasty-like, however, they become sclerosed in the course of the disease. Calcium deposits are often seen (that is ossifying myositis). They are typically revealed at X-ray examination of the muscles of the shoulder and pelvic girdles. Calcinosis in PM/DM is usually secondary and is often localized under the skin in the form of plaques or massive deposits in the areas of the shoulder and pelvic girdles where muscles are mostly affected. Areas of fascia and subcutaneous fat which are adjacent to the involved muscles are typically calcified.
Joint syndrome is less typical and is usually manifested by arthralgias or periarticular tissue involvement. Arthitis is rare; however, it is usually symmetric and non-destructive. Disorders of joint functions and the formation of contractures are typically associated with muscle involvement.
Internal organ involvement is seen in most patients with PM/DM but do not prevail in the clinical picture of the disease.
Pulmonary involvement is manifested by muscle weakness which usually spreads over the respiratory muscles, including diaphragm. It may be the cause of reduced respiratory function. Clinical manifestations include accelerated respiration and hypopnoe, inspiratory dyspnea, hypostatic pneumonia, marked pain syndrome. Pulmonary involvement may occur in the form of typical vascular or interstitial pneumonia (for example, pneumonitis, alveolitis) and aspiration pneumonia which is aggravated by respiratory muscle involvement. Pulmonary fibrosis with the signs of pulmonary hypertension and respiratory failure (for example, fibrosing alveolitis) may also occur. It is characterised by increased inspiratory dispnea, dry cough, crackling rales in the lower part of the lungs, pulmonary failure.
Cardiac involvement: myocardium is most commonaly affected; pericardium and endocardium – less commonly. Focal or diffuse myocarditis with dystrophic changes of the myocardium, cardiosclerosis, cardiofibrosis or arrhythmias may develop. Cases of dilated cardiomyopathy have been reported. Constrictive pericarditis is often seen in increased disease activity.
Renal involvement in DM/PM occurs quite rare and varies from focal glomerolonephritis or transient proteinuria to severe diffuse glomerolonephritis and myoglobinuria kidney with features of renal failure.
Gastrointestinal involvement is seen quite often and is manifested by increased dysphagia (due to involvement of the muscles of the fauces and the upper portion of the esophagus), loss of appetite, sometimes pain in the abdomen and symptoms of gastroenterocolitis. Dysphagia typically develops due to a reduction of contractile activity of pharyngeal muscles and muscles of the upper portion of the esophagus, disturbed peristalsis, muscle weakness of the soft palate and the tongue. Sometimes dysphagia may be the earliest symptom of the disease. Esophageal sphincter involvement typically results in reflux esophagitis. Severe form of progressive dysphagia when solid food is regurgitated and liquid food passes through the nose usually poses a threat to the patient’s life.
Neurological disturbances are not commonly seen and are manifested by various changes of sensititvity such as hyperaesthesia of peripheral or radicular type, hyperalgesia, paresthesia or areflexia. Sometimes pseudoneurological symptoms may occur. Poorly marked polyneuritis or even CNS involvement may develop due to vasculitis. Absence of reflexes in the involved extremities results from severe muscle involvement. Electromyography is commonly used to identify the cause of muscular changes.
Endocrine disturbances are quite rare.
Weight loss is considered to be the most common general symptom. Sometimes it may be rather significant. Fever is typically seen in an acute course of the disease or after an exacerbation of the disease. In most patients subfebrile temperature is typically seen.
Lab findings and other tests. Disease activity in DM/PM is characterised by mild anemia, leukocytosis, less commonly by leucopenia, eosinophilia, increased erythrocyte sedimentation rate (ESR), increased concentration of alfa-2 and gamma-globulin, seromucoid, ceruloplasmin, C-reactive protein.
Typical features of PM are elevated levels of muscle enzymes which are found in muscular tissue. They include creatine phosphokinase (CPK), fructose diphosphate aldolase, lactate dehydrogenase (LDG), aspartate and alanine aminotransferase.
Creatine phosphokinase levels may yield abnormal results. For example, its concentration may be 80 times as high as the normal one. Its concentration increases by 5 – 10 times in DM. However, some cases when patients don’t develop increased levels of creatine phosphokinase have been reported. In most patients elevated levels of serum myoglobin are typically revealed. Myoglobinuria is seen less commonly. Enzymeemia activity correlates with the extent of the disease activity and it may be helpful in the assessment of efficacy of treatment.
The range of autoantibodies revealed in patients depends on the type of PM/DM and the presence of concomitant diseases. In most (approximately 80 – 90%) patients with DM/PM antibodies to nuclear or cytolplasmic antigens (for example, Hep-2 cells) are typically present. Positive antinuclear antibodies with ring-like staining are common in patients with inflammatory myopathies.
Inflammatory myopathies induce formation of autoantibodies to myocytes. These antibodies are rarely revealed in other autoimmune diseases and are considered as myositis-specific. They may be divided into 4 groups. They are the following:
1. Antibodies to synthase of transfer RNA (anti-aminoacyl-tRNA-synthase). They take an active part in affection of skeletal muscles (antisynthase antibodies). They interact with cytolplasmic enzymes which mediate the link between aminoacids and transfer RNA. Antibodies to histidil-t-RNA-synthase are more frequent in patients with PM. In PM, especially in patients with interstitial pulmonary involvement, production of antisynthase antibodies is associated with the development of antisynthase syndrome.
2. Antibodies to signal recognition particles interact with one of the components of the cytoplasmic protein complex which is responsible for the transfer of polypeptides through the membranes of endoplasmic reticulum and block it.
3. Anti-Mi-2 antibodies interact with nucleoproteins whose function remains unknown. They do not combine with cytolplasmic proteins of myosin-specific antigens.
4. Antibodies to elongation factor i-alfa which provides transfer of aminoacyl-t-RNA-synthase to ribosomes and its movement along the polysome.
Sometimes muscle biopsy, either of the deltoid muscle or quadriceps, may enhance the clinician’s ability to diagnose DM. The disease which is revealed with the help of skin and muscle biopsy is not specific. It should be considered in diagnostics and differential diagnostics of the disease only in combination with clinical and laboratory findings of PM/DM.
Electromyography (EMG) enables the doctor to reveal reduced amplitude and shortened bioelectric potential of the affected muscles, polyphasy, sometimes – pathologic spontaneous activity such as fibrillation, pseudomyotonic disturbances, etc. EMG findings are not typical of DM/PM; they may vary in the course of the disease. Thus, they don’t enable the clinician to differentiate PM/DM from other myopathies. However, they are widely used in combination with other clinical findings and methods of examinatio
Diagnosis. No international criteria which can aid in the diagnosis and classification of DM/DM exist. However, in 1975 A. Bohan and J.B. Peter suggested a set of diagnostic criteria and in 1985 Т. Меdsger and А. Маsi suggested a set of classification criteria which are used in the diagnosis and classification of DM/DM. Seven diagnostic criteria of DM/PM are usually singled out. They are the following:
1) Common skin changes
2) Progressive proximal symmetrical muscle weakness (according to the patient’s past history and physical examination)
3) Elevated levels of one or more serum muscle enzymes
4) Abnormal (myopathic) findings on electromyogram (EMG)
5) Abnormal findings (polymyositis) on muscle biopsy
6) Increased creatinuria
7) Reduction of muscle weakness after corticosteroid therapy.
According to A Bohan and J.B. Peter, who suggested first five criteria, the presence of the first criterion or any other three criteria enables the doctor to make the diagnosis of DM. When the first criterion or any other two criteria are present, DM is considered to be likely. When the first criterion or some other criterion is present, DM is considered to be possible.
In PM 4 out of 5 criteria enables the clinician to make the diagnosis of certain PM. When 3 out of 4 criteria are present, PM is considered to be likely. When 2 out of 4 criteria are present, PM is considered to be possible.
These criteria were revised by Tahimoto et al. in 1995. They include the following:
1. Skin changes:
a) Heliotrope skin rash (light-violet erythema with edema of the upper eye-lids);
b) Gottron papules;
c) Erythema on the back surface of the joints of the extremities It is slightly raised. A slight scale may be present. Light-violet erythema in the knee and elbow joints is also common;
2. Proximal muscle weakness (upper or lower extremities and the trunk);
3. Elevated levels of creatine phosphokinase (CPK) or aldolase;
4. Pain in muscles when they are pressed or spontaneous pain;
5. Electromyogramm changes such as short polyphase bioelectric potential, fibrillation and pseudomyothic discharge);
6. Revealing of antisynthase antibodies;
7. Non-destructive arthritis or arthralgias;
8. Features of systemic inflammation (body temperature is usually higher than 37 C°, increased C-reactive protein or erythrocyte sedimentation rate levels – more than 20 mm/hour are present).
9. Myositis revealed in biopsy material (that is infiltration of skeletal muscles with inflammatory cells and focal or extensive degeneration of muscle fibers leading to necrosis and regenerative processes with irregular substitution of muscle fibers for fibrosis).
If any cutaneous manifestation and at least 4 other criteria are present, DM is highly possible. If 4 criteria from the second to the ninth are present, the diagnosis of PM is probable.
Differential diagnostics. Despite typical clinical manifestations of PM, its diagnostics, especially at an initial stage, may be somewhat difficult. PM should be differentiated from other diseases, which are associated with proximal muscle weakness and marked myalgias. PM should be differentiated from:
1. Infectious myositis
1) Viral myositis (coxsackieviruses, ECHO, influenza and HIV viruses)
2) Myositis in toxoplasmosis
3) Myositis in trichinosis
4) Myositis in cysticercosis
2. Metabolic myopathies
1) Acute alcohol myopathy
2) Mc Ardl’s disease
3) Carnitine-palmitil-transferase deficiency
4) Glicogenosis Type V
5) AMP-desaminase deficiency
3. Endocrine myopathies
1) Hypothyroidism
2) Hyperparathyroidism (primary and secondary)
3) Osteomalacia of any aetiology
4. Hypokalemia (prolonged administration of diuretics), administration of statins, hemofibrozil, epsilon-aminocapron acid and other drugs.
A number of diseases which should be differentiated from DM/PM are characterised by proximal muscle weakness and are not associated with myalgias. They are the following:
1. Demyelinating plyneuropathies
2. Intermittent porphyria
3. Myasthenia
4. Lamber-Iton’s syndrome
5. Spinal amyotrophy of Kugelberg-Welander
6. Progressive muscular dystrophies:
a) Extremity-girdle Erb’s disease
b) Facial-shoulder-scapular (myodystrophia Landuzi-Dejerine).
7. Hypercorticism, hyperthyroidism, acromegalia
8. Acid maltase deficiency
9. Chronic alcohol myopathy
10. Guillain-Barre’s syndrome
11. Drug-induced myopathies (caused by penicillamin, azothimidin, cimetidin, colchicines, glucocorticoids, cyclosporine, azathioprin, etc.)
It is important not only to diagnose PM/DM, but also to determine its clinical form and to perform differential diagnostics of primary (idiopathic) and secondary (tumorous) DM/PM.
PM/DM is often associated with rheumatic diseases. These diseases amount to 20% of all overlap syndromes. DM/PM is most commonly associated another connective- tissue disease or systemic scleroderma, rheumatoid arthritis or systemic lupus erythematosus. Less commonly DM/PM are associated with nodular periaatheriitis or rheumatism. It is usually diagnosed if clinical and laboratory findings of myositis and one or more connective-tissue diseases are present.
Treatment. Large doses of glucocorticoids (GC) help control muscle involvement in most patients. The minimum effective dose of GC is about 1mg/kg for children and adults. The earlier the treatment is started, the more effective it will be. Large doses of GC should be administered for 2 – 4 months until clearly marked signs of improvement appear. These signs usually include reduction of muscle weakness and facial edema, improvement of phonation, swallowing, appearance of pigmentation in the area of erythema as well as improvement of laboratory findings and muscle enzyme activity.
However, creatine phosphokinase activity doesn’t help decide on efficacy of treatment. Glucocorticoids in the dose of 1 mg/kg should be administered for 4 months before one decides on efficacy of treatment. A more prolonged period (not less than 6 months) is necessary for regeneration of muscle fibers. It should be considered when monitoring the patients and estimating efficacy of treatment.
There are different treatment and dosage options; however, the treatment of each patient should be strictly individualized. The doctor should evaluate the patient’s condition, efficacy of treatment, tolerance of the selected drug, etc. The maintenance dose should be selected and it should be administered for some years; however, in stable clinical remission the maintenance dose can be reduced or even withdrawn.
In increased clinical and laboratory disease activity, presence of visceral organ involvement and progressive myopathy pulse therapy with high doses of methylprednisolone (1000 mg) is typically administered. Methylprednisolone is usually administered intravenously with 0, 9% isotonic solution of sodium chloride (500 ml) or 5% glucose solution. The course of therapy lasts for 3 days.
Cytostatic drugs are also used in the treatment of PM/DM. Most experts believe that cytostatics are not effective without adequate glucocorticoid therapy. When cytostatic drugs are administered in combination with glucocorticoids, they have a “steroid-saving” action. It means that they give the possibility to achieve clinical effect with low doses of glucocorticoids). In DM/PM metotrexat, azathioprin, chlorbutin and cyclophosphamid are used .
Indications for administration of cytostatics are the following:
1. PM/DM resistant to maximum doses of glucocorticoids.
2. Presence of concomitant diseases or adverse effects, which limit the administration of glucocorticoids.
3. Unresponsiveness of some patients to glucocorticoid therapy.
Methotrexate which has few side effects and is characterised by low carcinogenicity compared with other cytotoxic drugs is the most beneficial.
Azathyoprin ranks the second, whereas alkylating agents such as Cyclophosphan, Chlorbutin should be indicated only if methotrexate and azathyoprin therapy was ineffective.
Oral methotrexate should be given in the dose of 7.5 mg to 25 – 30 mg weekly. Treatment should be started with a small dose. The dose should be gradually increased by 0.25 mg weekly until the optimum one. In patients who can`t take methotrexate orally, it should be given should be intravenously. Intravenous methotrexate should be started with 0.2 mg/kg once a week, increasing the dose by 0.2 mg/kg every 7 days.
Toxicity is evaluated after 6 days following the beginning of treatment and when the cumulative dose of 1500 mg is reached, liver biopsy is usually indicated. The dose of methotrexate should be reduced gradually, considering clinical and laboratory findings and creatine phosphokinase levels.
Azathyoprin isadministered in the dose of 2 – 3 mg/kg. Medical dose is 100 – 150 mg daily. The maximum clinical effect is typically revealed only 6 – 9 months after the beginning of treatment.
The maintainance dose is 50 mg daily. It should be decreased by 25 mg according to the same pattern as with methotrexate. According to Steinberg (1991), if methotrexate and azathyoprine are ineffective, combined treatment with 2 drugs should be undertaken.
Cyclophosphamide is rarely effective, but it is typically selected in interstitial pulmonary fibrosis. In PM/DM it should be used in patients who are unresponsive to methotrexate and azathyoprine. Cases of effective treatment with Chlorbutin (4 mg daily) in patients who received high doses of glucocorticoids, methotrexate and pulse therapy with methylprednisolone without marked clinical effect have been reported. There is evidence for successful use of Cyclosporine A in patients with juvenile DM and in adults with DM, especially in interstitial pulmonary fibrosis.








 Signs of regeneration of skeletal muscles are manifested by increased amounts of muscular nuclei as well as basophilia of muscle fibers. In chronic DM interstitial fibrous infiltrates often occur.
Signs of regeneration of skeletal muscles are manifested by increased amounts of muscular nuclei as well as basophilia of muscle fibers. In chronic DM interstitial fibrous infiltrates often occur.
 In a prolonged course of DM muscular atrophy resulting in the formation of dense connective tissue is often observed. Irreversible fibrous contractures typically develop.
Skin involvement is manifested by atrophy of papillae, thinning-out of the epidermis, thickening and structural changes of collagen fibers.
Classification:
In a prolonged course of DM muscular atrophy resulting in the formation of dense connective tissue is often observed. Irreversible fibrous contractures typically develop.
Skin involvement is manifested by atrophy of papillae, thinning-out of the epidermis, thickening and structural changes of collagen fibers.
Classification:
- Primary PM
- Primary DM
- DM or PM associated with malignancy
- Juvenile DM or PM
- PM or DM associated with another connective-tissue diseases
 Erythema in DM is quite stable and is often associated with edema, which is sometimes similar to Quincke’s edema, especially at an initial stage of the disease.
Erythema in DM is quite stable and is often associated with edema, which is sometimes similar to Quincke’s edema, especially at an initial stage of the disease.
 Soft tissues are typically enlarged, pasty-like when palpated, covered with pruritic erythema; less commonly they are associated with hemorrhages, telangiectasias, ulcers and hyperkeratosis. Pathognomonic changes occurring in DM include heliotrope (“sun”) eye-lids (that is stained violet and swollen eye-lids) and Gottron papules consisting of slightly elevated violaceous papules and plaques over finger joints.
Soft tissues are typically enlarged, pasty-like when palpated, covered with pruritic erythema; less commonly they are associated with hemorrhages, telangiectasias, ulcers and hyperkeratosis. Pathognomonic changes occurring in DM include heliotrope (“sun”) eye-lids (that is stained violet and swollen eye-lids) and Gottron papules consisting of slightly elevated violaceous papules and plaques over finger joints.
 Other symptoms include “mechanic’s hands”, that is hyperkeratotic eruptions over the fingers and palms and “shawl sign”, that is a V-shaped erythema over the anterior neck and upper chest and back. In the course of DM erythema and edema spread over other parts of the body. Atrophy, pigmentation and dryness of skin usually aggaravate.
Other symptoms include “mechanic’s hands”, that is hyperkeratotic eruptions over the fingers and palms and “shawl sign”, that is a V-shaped erythema over the anterior neck and upper chest and back. In the course of DM erythema and edema spread over other parts of the body. Atrophy, pigmentation and dryness of skin usually aggaravate.
 Foci of skin depigmentation may occur. Nail trophic changes and hair loss may be observed. Mucous membranes may be also involved leading to conjunctivitis, stomatitis, edema of fauces and true vocal cords.
Sometimes Raynaud’s syndrome may be present. It usually has a two-phase course and doesn’t cause trophic ulcers. Although cases of periungual bed infarction, petechia and livedo reticularis have been reported.
Muscular syndrome is the key syndrome in diagnosis of DM/PM. Severe, sometimes necrotic myositis of proximal muscles of the extremities is typical of DM. Muscle weakness is usually slowly progressive.
Foci of skin depigmentation may occur. Nail trophic changes and hair loss may be observed. Mucous membranes may be also involved leading to conjunctivitis, stomatitis, edema of fauces and true vocal cords.
Sometimes Raynaud’s syndrome may be present. It usually has a two-phase course and doesn’t cause trophic ulcers. Although cases of periungual bed infarction, petechia and livedo reticularis have been reported.
Muscular syndrome is the key syndrome in diagnosis of DM/PM. Severe, sometimes necrotic myositis of proximal muscles of the extremities is typical of DM. Muscle weakness is usually slowly progressive.
 However, in some cases the disease may have an acute course. In this case it is characterised by a rapid development of acute respiratory and renal (myoglobinuria) failure. Muscle weakness, especially muscles of the pelvic girdle and the thigh is considered to be the primary manifestation of DM. However, the earliest symptoms may include dysphagia and weakness of neck muscles. Symmetric proximal muscle weakness of the extremities is typical. Patients often begin to note weakness of muscles when combing their hair, dressing themselves, climbing stairs, walking, rising from a sitting or lying position, though the movements of hands, fingers and feet may be quite normal.
Larynx and pharynx muscle involvement typically results in dysphagia, nasality (nasal intonation), sometimes aphonia, hoarseness, choking on swallowing. Aspiration of food into the trachea is also possible. Patients with PM/DM have difficulties in swallowing solid and liquid food which sometimes passes through the nose. In DM/PM the upper portion of the esophagus, muscles of the soft palate and the tongue are usually involved.
In case of ocular muscle involvement diplopia and ptosis may develop. When facial muscles are involved, the person may develop a mask-like face. Sphincter involvement typically results in sphincter incompetence, that is involuntary defecation and urination. Intercostal muscle and diaphragm involvement may result in disturbed respiration.
Muscles are usually tender on palpation, swollen, pasty-like, however, they become sclerosed in the course of the disease. Calcium deposits are often seen (that is ossifying myositis). They are typically revealed at X-ray examination of the muscles of the shoulder and pelvic girdles. Calcinosis in PM/DM is usually secondary and is often localized under the skin in the form of plaques or massive deposits in the areas of the shoulder and pelvic girdles where muscles are mostly affected. Areas of fascia and subcutaneous fat which are adjacent to the involved muscles are typically calcified.
Joint syndrome is less typical and is usually manifested by arthralgias or periarticular tissue involvement. Arthitis is rare; however, it is usually symmetric and non-destructive. Disorders of joint functions and the formation of contractures are typically associated with muscle involvement.
Internal organ involvement is seen in most patients with PM/DM but do not prevail in the clinical picture of the disease.
Pulmonary involvement is manifested by muscle weakness which usually spreads over the respiratory muscles, including diaphragm. It may be the cause of reduced respiratory function. Clinical manifestations include accelerated respiration and hypopnoe, inspiratory dyspnea, hypostatic pneumonia, marked pain syndrome. Pulmonary involvement may occur in the form of typical vascular or interstitial pneumonia (for example, pneumonitis, alveolitis) and aspiration pneumonia which is aggravated by respiratory muscle involvement. Pulmonary fibrosis with the signs of pulmonary hypertension and respiratory failure (for example, fibrosing alveolitis) may also occur. It is characterised by increased inspiratory dispnea, dry cough, crackling rales in the lower part of the lungs, pulmonary failure.
Cardiac involvement: myocardium is most commonaly affected; pericardium and endocardium – less commonly. Focal or diffuse myocarditis with dystrophic changes of the myocardium, cardiosclerosis, cardiofibrosis or arrhythmias may develop. Cases of dilated cardiomyopathy have been reported. Constrictive pericarditis is often seen in increased disease activity.
Renal involvement in DM/PM occurs quite rare and varies from focal glomerolonephritis or transient proteinuria to severe diffuse glomerolonephritis and myoglobinuria kidney with features of renal failure.
Gastrointestinal involvement is seen quite often and is manifested by increased dysphagia (due to involvement of the muscles of the fauces and the upper portion of the esophagus), loss of appetite, sometimes pain in the abdomen and symptoms of gastroenterocolitis. Dysphagia typically develops due to a reduction of contractile activity of pharyngeal muscles and muscles of the upper portion of the esophagus, disturbed peristalsis, muscle weakness of the soft palate and the tongue. Sometimes dysphagia may be the earliest symptom of the disease. Esophageal sphincter involvement typically results in reflux esophagitis. Severe form of progressive dysphagia when solid food is regurgitated and liquid food passes through the nose usually poses a threat to the patient’s life.
Neurological disturbances are not commonly seen and are manifested by various changes of sensititvity such as hyperaesthesia of peripheral or radicular type, hyperalgesia, paresthesia or areflexia. Sometimes pseudoneurological symptoms may occur. Poorly marked polyneuritis or even CNS involvement may develop due to vasculitis. Absence of reflexes in the involved extremities results from severe muscle involvement. Electromyography is commonly used to identify the cause of muscular changes.
Endocrine disturbances are quite rare.
Weight loss is considered to be the most common general symptom. Sometimes it may be rather significant. Fever is typically seen in an acute course of the disease or after an exacerbation of the disease. In most patients subfebrile temperature is typically seen.
Lab findings and other tests. Disease activity in DM/PM is characterised by mild anemia, leukocytosis, less commonly by leucopenia, eosinophilia, increased erythrocyte sedimentation rate (ESR), increased concentration of alfa-2 and gamma-globulin, seromucoid, ceruloplasmin, C-reactive protein.
Typical features of PM are elevated levels of muscle enzymes which are found in muscular tissue. They include creatine phosphokinase (CPK), fructose diphosphate aldolase, lactate dehydrogenase (LDG), aspartate and alanine aminotransferase.
Creatine phosphokinase levels may yield abnormal results. For example, its concentration may be 80 times as high as the normal one. Its concentration increases by 5 – 10 times in DM. However, some cases when patients don’t develop increased levels of creatine phosphokinase have been reported. In most patients elevated levels of serum myoglobin are typically revealed. Myoglobinuria is seen less commonly. Enzymeemia activity correlates with the extent of the disease activity and it may be helpful in the assessment of efficacy of treatment.
The range of autoantibodies revealed in patients depends on the type of PM/DM and the presence of concomitant diseases. In most (approximately 80 – 90%) patients with DM/PM antibodies to nuclear or cytolplasmic antigens (for example, Hep-2 cells) are typically present. Positive antinuclear antibodies with ring-like staining are common in patients with inflammatory myopathies.
Inflammatory myopathies induce formation of autoantibodies to myocytes. These antibodies are rarely revealed in other autoimmune diseases and are considered as myositis-specific. They may be divided into 4 groups. They are the following:
1. Antibodies to synthase of transfer RNA (anti-aminoacyl-tRNA-synthase). They take an active part in affection of skeletal muscles (antisynthase antibodies). They interact with cytolplasmic enzymes which mediate the link between aminoacids and transfer RNA. Antibodies to histidil-t-RNA-synthase are more frequent in patients with PM. In PM, especially in patients with interstitial pulmonary involvement, production of antisynthase antibodies is associated with the development of antisynthase syndrome.
2. Antibodies to signal recognition particles interact with one of the components of the cytoplasmic protein complex which is responsible for the transfer of polypeptides through the membranes of endoplasmic reticulum and block it.
3. Anti-Mi-2 antibodies interact with nucleoproteins whose function remains unknown. They do not combine with cytolplasmic proteins of myosin-specific antigens.
4. Antibodies to elongation factor i-alfa which provides transfer of aminoacyl-t-RNA-synthase to ribosomes and its movement along the polysome.
Sometimes muscle biopsy, either of the deltoid muscle or quadriceps, may enhance the clinician’s ability to diagnose DM. The disease which is revealed with the help of skin and muscle biopsy is not specific. It should be considered in diagnostics and differential diagnostics of the disease only in combination with clinical and laboratory findings of PM/DM.
Electromyography (EMG) enables the doctor to reveal reduced amplitude and shortened bioelectric potential of the affected muscles, polyphasy, sometimes – pathologic spontaneous activity such as fibrillation, pseudomyotonic disturbances, etc. EMG findings are not typical of DM/PM; they may vary in the course of the disease. Thus, they don’t enable the clinician to differentiate PM/DM from other myopathies. However, they are widely used in combination with other clinical findings and methods of examinatio
Diagnosis. No international criteria which can aid in the diagnosis and classification of DM/DM exist. However, in 1975 A. Bohan and J.B. Peter suggested a set of diagnostic criteria and in 1985 Т. Меdsger and А. Маsi suggested a set of classification criteria which are used in the diagnosis and classification of DM/DM. Seven diagnostic criteria of DM/PM are usually singled out. They are the following:
1) Common skin changes
2) Progressive proximal symmetrical muscle weakness (according to the patient’s past history and physical examination)
3) Elevated levels of one or more serum muscle enzymes
4) Abnormal (myopathic) findings on electromyogram (EMG)
5) Abnormal findings (polymyositis) on muscle biopsy
6) Increased creatinuria
7) Reduction of muscle weakness after corticosteroid therapy.
According to A Bohan and J.B. Peter, who suggested first five criteria, the presence of the first criterion or any other three criteria enables the doctor to make the diagnosis of DM. When the first criterion or any other two criteria are present, DM is considered to be likely. When the first criterion or some other criterion is present, DM is considered to be possible.
In PM 4 out of 5 criteria enables the clinician to make the diagnosis of certain PM. When 3 out of 4 criteria are present, PM is considered to be likely. When 2 out of 4 criteria are present, PM is considered to be possible.
These criteria were revised by Tahimoto et al. in 1995. They include the following:
1. Skin changes:
a) Heliotrope skin rash (light-violet erythema with edema of the upper eye-lids);
b) Gottron papules;
c) Erythema on the back surface of the joints of the extremities It is slightly raised. A slight scale may be present. Light-violet erythema in the knee and elbow joints is also common;
2. Proximal muscle weakness (upper or lower extremities and the trunk);
3. Elevated levels of creatine phosphokinase (CPK) or aldolase;
4. Pain in muscles when they are pressed or spontaneous pain;
5. Electromyogramm changes such as short polyphase bioelectric potential, fibrillation and pseudomyothic discharge);
6. Revealing of antisynthase antibodies;
7. Non-destructive arthritis or arthralgias;
8. Features of systemic inflammation (body temperature is usually higher than 37 C°, increased C-reactive protein or erythrocyte sedimentation rate levels – more than 20 mm/hour are present).
9. Myositis revealed in biopsy material (that is infiltration of skeletal muscles with inflammatory cells and focal or extensive degeneration of muscle fibers leading to necrosis and regenerative processes with irregular substitution of muscle fibers for fibrosis).
If any cutaneous manifestation and at least 4 other criteria are present, DM is highly possible. If 4 criteria from the second to the ninth are present, the diagnosis of PM is probable.
Differential diagnostics. Despite typical clinical manifestations of PM, its diagnostics, especially at an initial stage, may be somewhat difficult. PM should be differentiated from other diseases, which are associated with proximal muscle weakness and marked myalgias. PM should be differentiated from:
1. Infectious myositis
1) Viral myositis (coxsackieviruses, ECHO, influenza and HIV viruses)
2) Myositis in toxoplasmosis
3) Myositis in trichinosis
4) Myositis in cysticercosis
2. Metabolic myopathies
1) Acute alcohol myopathy
2) Mc Ardl’s disease
3) Carnitine-palmitil-transferase deficiency
4) Glicogenosis Type V
5) AMP-desaminase deficiency
3. Endocrine myopathies
1) Hypothyroidism
2) Hyperparathyroidism (primary and secondary)
3) Osteomalacia of any aetiology
4. Hypokalemia (prolonged administration of diuretics), administration of statins, hemofibrozil, epsilon-aminocapron acid and other drugs.
A number of diseases which should be differentiated from DM/PM are characterised by proximal muscle weakness and are not associated with myalgias. They are the following:
1. Demyelinating plyneuropathies
2. Intermittent porphyria
3. Myasthenia
4. Lamber-Iton’s syndrome
5. Spinal amyotrophy of Kugelberg-Welander
6. Progressive muscular dystrophies:
a) Extremity-girdle Erb’s disease
b) Facial-shoulder-scapular (myodystrophia Landuzi-Dejerine).
7. Hypercorticism, hyperthyroidism, acromegalia
8. Acid maltase deficiency
9. Chronic alcohol myopathy
10. Guillain-Barre’s syndrome
11. Drug-induced myopathies (caused by penicillamin, azothimidin, cimetidin, colchicines, glucocorticoids, cyclosporine, azathioprin, etc.)
It is important not only to diagnose PM/DM, but also to determine its clinical form and to perform differential diagnostics of primary (idiopathic) and secondary (tumorous) DM/PM.
PM/DM is often associated with rheumatic diseases. These diseases amount to 20% of all overlap syndromes. DM/PM is most commonly associated another connective- tissue disease or systemic scleroderma, rheumatoid arthritis or systemic lupus erythematosus. Less commonly DM/PM are associated with nodular periaatheriitis or rheumatism. It is usually diagnosed if clinical and laboratory findings of myositis and one or more connective-tissue diseases are present.
Treatment. Large doses of glucocorticoids (GC) help control muscle involvement in most patients. The minimum effective dose of GC is about 1mg/kg for children and adults. The earlier the treatment is started, the more effective it will be. Large doses of GC should be administered for 2 – 4 months until clearly marked signs of improvement appear. These signs usually include reduction of muscle weakness and facial edema, improvement of phonation, swallowing, appearance of pigmentation in the area of erythema as well as improvement of laboratory findings and muscle enzyme activity.
However, creatine phosphokinase activity doesn’t help decide on efficacy of treatment. Glucocorticoids in the dose of 1 mg/kg should be administered for 4 months before one decides on efficacy of treatment. A more prolonged period (not less than 6 months) is necessary for regeneration of muscle fibers. It should be considered when monitoring the patients and estimating efficacy of treatment.
There are different treatment and dosage options; however, the treatment of each patient should be strictly individualized. The doctor should evaluate the patient’s condition, efficacy of treatment, tolerance of the selected drug, etc. The maintenance dose should be selected and it should be administered for some years; however, in stable clinical remission the maintenance dose can be reduced or even withdrawn.
In increased clinical and laboratory disease activity, presence of visceral organ involvement and progressive myopathy pulse therapy with high doses of methylprednisolone (1000 mg) is typically administered. Methylprednisolone is usually administered intravenously with 0, 9% isotonic solution of sodium chloride (500 ml) or 5% glucose solution. The course of therapy lasts for 3 days.
Cytostatic drugs are also used in the treatment of PM/DM. Most experts believe that cytostatics are not effective without adequate glucocorticoid therapy. When cytostatic drugs are administered in combination with glucocorticoids, they have a “steroid-saving” action. It means that they give the possibility to achieve clinical effect with low doses of glucocorticoids). In DM/PM metotrexat, azathioprin, chlorbutin and cyclophosphamid are used .
Indications for administration of cytostatics are the following:
1. PM/DM resistant to maximum doses of glucocorticoids.
2. Presence of concomitant diseases or adverse effects, which limit the administration of glucocorticoids.
3. Unresponsiveness of some patients to glucocorticoid therapy.
Methotrexate which has few side effects and is characterised by low carcinogenicity compared with other cytotoxic drugs is the most beneficial.
Azathyoprin ranks the second, whereas alkylating agents such as Cyclophosphan, Chlorbutin should be indicated only if methotrexate and azathyoprin therapy was ineffective.
Oral methotrexate should be given in the dose of 7.5 mg to 25 – 30 mg weekly. Treatment should be started with a small dose. The dose should be gradually increased by 0.25 mg weekly until the optimum one. In patients who can`t take methotrexate orally, it should be given should be intravenously. Intravenous methotrexate should be started with 0.2 mg/kg once a week, increasing the dose by 0.2 mg/kg every 7 days.
Toxicity is evaluated after 6 days following the beginning of treatment and when the cumulative dose of 1500 mg is reached, liver biopsy is usually indicated. The dose of methotrexate should be reduced gradually, considering clinical and laboratory findings and creatine phosphokinase levels.
Azathyoprin isadministered in the dose of 2 – 3 mg/kg. Medical dose is 100 – 150 mg daily. The maximum clinical effect is typically revealed only 6 – 9 months after the beginning of treatment.
The maintainance dose is 50 mg daily. It should be decreased by 25 mg according to the same pattern as with methotrexate. According to Steinberg (1991), if methotrexate and azathyoprine are ineffective, combined treatment with 2 drugs should be undertaken.
Cyclophosphamide is rarely effective, but it is typically selected in interstitial pulmonary fibrosis. In PM/DM it should be used in patients who are unresponsive to methotrexate and azathyoprine. Cases of effective treatment with Chlorbutin (4 mg daily) in patients who received high doses of glucocorticoids, methotrexate and pulse therapy with methylprednisolone without marked clinical effect have been reported. There is evidence for successful use of Cyclosporine A in patients with juvenile DM and in adults with DM, especially in interstitial pulmonary fibrosis.
However, in some cases the disease may have an acute course. In this case it is characterised by a rapid development of acute respiratory and renal (myoglobinuria) failure. Muscle weakness, especially muscles of the pelvic girdle and the thigh is considered to be the primary manifestation of DM. However, the earliest symptoms may include dysphagia and weakness of neck muscles. Symmetric proximal muscle weakness of the extremities is typical. Patients often begin to note weakness of muscles when combing their hair, dressing themselves, climbing stairs, walking, rising from a sitting or lying position, though the movements of hands, fingers and feet may be quite normal.
Larynx and pharynx muscle involvement typically results in dysphagia, nasality (nasal intonation), sometimes aphonia, hoarseness, choking on swallowing. Aspiration of food into the trachea is also possible. Patients with PM/DM have difficulties in swallowing solid and liquid food which sometimes passes through the nose. In DM/PM the upper portion of the esophagus, muscles of the soft palate and the tongue are usually involved.
In case of ocular muscle involvement diplopia and ptosis may develop. When facial muscles are involved, the person may develop a mask-like face. Sphincter involvement typically results in sphincter incompetence, that is involuntary defecation and urination. Intercostal muscle and diaphragm involvement may result in disturbed respiration.
Muscles are usually tender on palpation, swollen, pasty-like, however, they become sclerosed in the course of the disease. Calcium deposits are often seen (that is ossifying myositis). They are typically revealed at X-ray examination of the muscles of the shoulder and pelvic girdles. Calcinosis in PM/DM is usually secondary and is often localized under the skin in the form of plaques or massive deposits in the areas of the shoulder and pelvic girdles where muscles are mostly affected. Areas of fascia and subcutaneous fat which are adjacent to the involved muscles are typically calcified.
Joint syndrome is less typical and is usually manifested by arthralgias or periarticular tissue involvement. Arthitis is rare; however, it is usually symmetric and non-destructive. Disorders of joint functions and the formation of contractures are typically associated with muscle involvement.
Internal organ involvement is seen in most patients with PM/DM but do not prevail in the clinical picture of the disease.
Pulmonary involvement is manifested by muscle weakness which usually spreads over the respiratory muscles, including diaphragm. It may be the cause of reduced respiratory function. Clinical manifestations include accelerated respiration and hypopnoe, inspiratory dyspnea, hypostatic pneumonia, marked pain syndrome. Pulmonary involvement may occur in the form of typical vascular or interstitial pneumonia (for example, pneumonitis, alveolitis) and aspiration pneumonia which is aggravated by respiratory muscle involvement. Pulmonary fibrosis with the signs of pulmonary hypertension and respiratory failure (for example, fibrosing alveolitis) may also occur. It is characterised by increased inspiratory dispnea, dry cough, crackling rales in the lower part of the lungs, pulmonary failure.
Cardiac involvement: myocardium is most commonaly affected; pericardium and endocardium – less commonly. Focal or diffuse myocarditis with dystrophic changes of the myocardium, cardiosclerosis, cardiofibrosis or arrhythmias may develop. Cases of dilated cardiomyopathy have been reported. Constrictive pericarditis is often seen in increased disease activity.
Renal involvement in DM/PM occurs quite rare and varies from focal glomerolonephritis or transient proteinuria to severe diffuse glomerolonephritis and myoglobinuria kidney with features of renal failure.
Gastrointestinal involvement is seen quite often and is manifested by increased dysphagia (due to involvement of the muscles of the fauces and the upper portion of the esophagus), loss of appetite, sometimes pain in the abdomen and symptoms of gastroenterocolitis. Dysphagia typically develops due to a reduction of contractile activity of pharyngeal muscles and muscles of the upper portion of the esophagus, disturbed peristalsis, muscle weakness of the soft palate and the tongue. Sometimes dysphagia may be the earliest symptom of the disease. Esophageal sphincter involvement typically results in reflux esophagitis. Severe form of progressive dysphagia when solid food is regurgitated and liquid food passes through the nose usually poses a threat to the patient’s life.
Neurological disturbances are not commonly seen and are manifested by various changes of sensititvity such as hyperaesthesia of peripheral or radicular type, hyperalgesia, paresthesia or areflexia. Sometimes pseudoneurological symptoms may occur. Poorly marked polyneuritis or even CNS involvement may develop due to vasculitis. Absence of reflexes in the involved extremities results from severe muscle involvement. Electromyography is commonly used to identify the cause of muscular changes.
Endocrine disturbances are quite rare.
Weight loss is considered to be the most common general symptom. Sometimes it may be rather significant. Fever is typically seen in an acute course of the disease or after an exacerbation of the disease. In most patients subfebrile temperature is typically seen.
Lab findings and other tests. Disease activity in DM/PM is characterised by mild anemia, leukocytosis, less commonly by leucopenia, eosinophilia, increased erythrocyte sedimentation rate (ESR), increased concentration of alfa-2 and gamma-globulin, seromucoid, ceruloplasmin, C-reactive protein.
Typical features of PM are elevated levels of muscle enzymes which are found in muscular tissue. They include creatine phosphokinase (CPK), fructose diphosphate aldolase, lactate dehydrogenase (LDG), aspartate and alanine aminotransferase.
Creatine phosphokinase levels may yield abnormal results. For example, its concentration may be 80 times as high as the normal one. Its concentration increases by 5 – 10 times in DM. However, some cases when patients don’t develop increased levels of creatine phosphokinase have been reported. In most patients elevated levels of serum myoglobin are typically revealed. Myoglobinuria is seen less commonly. Enzymeemia activity correlates with the extent of the disease activity and it may be helpful in the assessment of efficacy of treatment.
The range of autoantibodies revealed in patients depends on the type of PM/DM and the presence of concomitant diseases. In most (approximately 80 – 90%) patients with DM/PM antibodies to nuclear or cytolplasmic antigens (for example, Hep-2 cells) are typically present. Positive antinuclear antibodies with ring-like staining are common in patients with inflammatory myopathies.
Inflammatory myopathies induce formation of autoantibodies to myocytes. These antibodies are rarely revealed in other autoimmune diseases and are considered as myositis-specific. They may be divided into 4 groups. They are the following:
1. Antibodies to synthase of transfer RNA (anti-aminoacyl-tRNA-synthase). They take an active part in affection of skeletal muscles (antisynthase antibodies). They interact with cytolplasmic enzymes which mediate the link between aminoacids and transfer RNA. Antibodies to histidil-t-RNA-synthase are more frequent in patients with PM. In PM, especially in patients with interstitial pulmonary involvement, production of antisynthase antibodies is associated with the development of antisynthase syndrome.
2. Antibodies to signal recognition particles interact with one of the components of the cytoplasmic protein complex which is responsible for the transfer of polypeptides through the membranes of endoplasmic reticulum and block it.
3. Anti-Mi-2 antibodies interact with nucleoproteins whose function remains unknown. They do not combine with cytolplasmic proteins of myosin-specific antigens.
4. Antibodies to elongation factor i-alfa which provides transfer of aminoacyl-t-RNA-synthase to ribosomes and its movement along the polysome.
Sometimes muscle biopsy, either of the deltoid muscle or quadriceps, may enhance the clinician’s ability to diagnose DM. The disease which is revealed with the help of skin and muscle biopsy is not specific. It should be considered in diagnostics and differential diagnostics of the disease only in combination with clinical and laboratory findings of PM/DM.
Electromyography (EMG) enables the doctor to reveal reduced amplitude and shortened bioelectric potential of the affected muscles, polyphasy, sometimes – pathologic spontaneous activity such as fibrillation, pseudomyotonic disturbances, etc. EMG findings are not typical of DM/PM; they may vary in the course of the disease. Thus, they don’t enable the clinician to differentiate PM/DM from other myopathies. However, they are widely used in combination with other clinical findings and methods of examinatio
Diagnosis. No international criteria which can aid in the diagnosis and classification of DM/DM exist. However, in 1975 A. Bohan and J.B. Peter suggested a set of diagnostic criteria and in 1985 Т. Меdsger and А. Маsi suggested a set of classification criteria which are used in the diagnosis and classification of DM/DM. Seven diagnostic criteria of DM/PM are usually singled out. They are the following:
1) Common skin changes
2) Progressive proximal symmetrical muscle weakness (according to the patient’s past history and physical examination)
3) Elevated levels of one or more serum muscle enzymes
4) Abnormal (myopathic) findings on electromyogram (EMG)
5) Abnormal findings (polymyositis) on muscle biopsy
6) Increased creatinuria
7) Reduction of muscle weakness after corticosteroid therapy.
According to A Bohan and J.B. Peter, who suggested first five criteria, the presence of the first criterion or any other three criteria enables the doctor to make the diagnosis of DM. When the first criterion or any other two criteria are present, DM is considered to be likely. When the first criterion or some other criterion is present, DM is considered to be possible.
In PM 4 out of 5 criteria enables the clinician to make the diagnosis of certain PM. When 3 out of 4 criteria are present, PM is considered to be likely. When 2 out of 4 criteria are present, PM is considered to be possible.
These criteria were revised by Tahimoto et al. in 1995. They include the following:
1. Skin changes:
a) Heliotrope skin rash (light-violet erythema with edema of the upper eye-lids);
b) Gottron papules;
c) Erythema on the back surface of the joints of the extremities It is slightly raised. A slight scale may be present. Light-violet erythema in the knee and elbow joints is also common;
2. Proximal muscle weakness (upper or lower extremities and the trunk);
3. Elevated levels of creatine phosphokinase (CPK) or aldolase;
4. Pain in muscles when they are pressed or spontaneous pain;
5. Electromyogramm changes such as short polyphase bioelectric potential, fibrillation and pseudomyothic discharge);
6. Revealing of antisynthase antibodies;
7. Non-destructive arthritis or arthralgias;
8. Features of systemic inflammation (body temperature is usually higher than 37 C°, increased C-reactive protein or erythrocyte sedimentation rate levels – more than 20 mm/hour are present).
9. Myositis revealed in biopsy material (that is infiltration of skeletal muscles with inflammatory cells and focal or extensive degeneration of muscle fibers leading to necrosis and regenerative processes with irregular substitution of muscle fibers for fibrosis).
If any cutaneous manifestation and at least 4 other criteria are present, DM is highly possible. If 4 criteria from the second to the ninth are present, the diagnosis of PM is probable.
Differential diagnostics. Despite typical clinical manifestations of PM, its diagnostics, especially at an initial stage, may be somewhat difficult. PM should be differentiated from other diseases, which are associated with proximal muscle weakness and marked myalgias. PM should be differentiated from:
1. Infectious myositis
1) Viral myositis (coxsackieviruses, ECHO, influenza and HIV viruses)
2) Myositis in toxoplasmosis
3) Myositis in trichinosis
4) Myositis in cysticercosis
2. Metabolic myopathies
1) Acute alcohol myopathy
2) Mc Ardl’s disease
3) Carnitine-palmitil-transferase deficiency
4) Glicogenosis Type V
5) AMP-desaminase deficiency
3. Endocrine myopathies
1) Hypothyroidism
2) Hyperparathyroidism (primary and secondary)
3) Osteomalacia of any aetiology
4. Hypokalemia (prolonged administration of diuretics), administration of statins, hemofibrozil, epsilon-aminocapron acid and other drugs.
A number of diseases which should be differentiated from DM/PM are characterised by proximal muscle weakness and are not associated with myalgias. They are the following:
1. Demyelinating plyneuropathies
2. Intermittent porphyria
3. Myasthenia
4. Lamber-Iton’s syndrome
5. Spinal amyotrophy of Kugelberg-Welander
6. Progressive muscular dystrophies:
a) Extremity-girdle Erb’s disease
b) Facial-shoulder-scapular (myodystrophia Landuzi-Dejerine).
7. Hypercorticism, hyperthyroidism, acromegalia
8. Acid maltase deficiency
9. Chronic alcohol myopathy
10. Guillain-Barre’s syndrome
11. Drug-induced myopathies (caused by penicillamin, azothimidin, cimetidin, colchicines, glucocorticoids, cyclosporine, azathioprin, etc.)
It is important not only to diagnose PM/DM, but also to determine its clinical form and to perform differential diagnostics of primary (idiopathic) and secondary (tumorous) DM/PM.
PM/DM is often associated with rheumatic diseases. These diseases amount to 20% of all overlap syndromes. DM/PM is most commonly associated another connective- tissue disease or systemic scleroderma, rheumatoid arthritis or systemic lupus erythematosus. Less commonly DM/PM are associated with nodular periaatheriitis or rheumatism. It is usually diagnosed if clinical and laboratory findings of myositis and one or more connective-tissue diseases are present.
Treatment. Large doses of glucocorticoids (GC) help control muscle involvement in most patients. The minimum effective dose of GC is about 1mg/kg for children and adults. The earlier the treatment is started, the more effective it will be. Large doses of GC should be administered for 2 – 4 months until clearly marked signs of improvement appear. These signs usually include reduction of muscle weakness and facial edema, improvement of phonation, swallowing, appearance of pigmentation in the area of erythema as well as improvement of laboratory findings and muscle enzyme activity.
However, creatine phosphokinase activity doesn’t help decide on efficacy of treatment. Glucocorticoids in the dose of 1 mg/kg should be administered for 4 months before one decides on efficacy of treatment. A more prolonged period (not less than 6 months) is necessary for regeneration of muscle fibers. It should be considered when monitoring the patients and estimating efficacy of treatment.
There are different treatment and dosage options; however, the treatment of each patient should be strictly individualized. The doctor should evaluate the patient’s condition, efficacy of treatment, tolerance of the selected drug, etc. The maintenance dose should be selected and it should be administered for some years; however, in stable clinical remission the maintenance dose can be reduced or even withdrawn.
In increased clinical and laboratory disease activity, presence of visceral organ involvement and progressive myopathy pulse therapy with high doses of methylprednisolone (1000 mg) is typically administered. Methylprednisolone is usually administered intravenously with 0, 9% isotonic solution of sodium chloride (500 ml) or 5% glucose solution. The course of therapy lasts for 3 days.
Cytostatic drugs are also used in the treatment of PM/DM. Most experts believe that cytostatics are not effective without adequate glucocorticoid therapy. When cytostatic drugs are administered in combination with glucocorticoids, they have a “steroid-saving” action. It means that they give the possibility to achieve clinical effect with low doses of glucocorticoids). In DM/PM metotrexat, azathioprin, chlorbutin and cyclophosphamid are used .
Indications for administration of cytostatics are the following:
1. PM/DM resistant to maximum doses of glucocorticoids.
2. Presence of concomitant diseases or adverse effects, which limit the administration of glucocorticoids.
3. Unresponsiveness of some patients to glucocorticoid therapy.
Methotrexate which has few side effects and is characterised by low carcinogenicity compared with other cytotoxic drugs is the most beneficial.
Azathyoprin ranks the second, whereas alkylating agents such as Cyclophosphan, Chlorbutin should be indicated only if methotrexate and azathyoprin therapy was ineffective.
Oral methotrexate should be given in the dose of 7.5 mg to 25 – 30 mg weekly. Treatment should be started with a small dose. The dose should be gradually increased by 0.25 mg weekly until the optimum one. In patients who can`t take methotrexate orally, it should be given should be intravenously. Intravenous methotrexate should be started with 0.2 mg/kg once a week, increasing the dose by 0.2 mg/kg every 7 days.
Toxicity is evaluated after 6 days following the beginning of treatment and when the cumulative dose of 1500 mg is reached, liver biopsy is usually indicated. The dose of methotrexate should be reduced gradually, considering clinical and laboratory findings and creatine phosphokinase levels.
Azathyoprin isadministered in the dose of 2 – 3 mg/kg. Medical dose is 100 – 150 mg daily. The maximum clinical effect is typically revealed only 6 – 9 months after the beginning of treatment.
The maintainance dose is 50 mg daily. It should be decreased by 25 mg according to the same pattern as with methotrexate. According to Steinberg (1991), if methotrexate and azathyoprine are ineffective, combined treatment with 2 drugs should be undertaken.
Cyclophosphamide is rarely effective, but it is typically selected in interstitial pulmonary fibrosis. In PM/DM it should be used in patients who are unresponsive to methotrexate and azathyoprine. Cases of effective treatment with Chlorbutin (4 mg daily) in patients who received high doses of glucocorticoids, methotrexate and pulse therapy with methylprednisolone without marked clinical effect have been reported. There is evidence for successful use of Cyclosporine A in patients with juvenile DM and in adults with DM, especially in interstitial pulmonary fibrosis.




