Просмотров: 3 060
Laboratory diagnosis and control of inflammation in rheumatology. Addition
Ph.D. Khanov A.G.
Laboratory control of inflammation in rheumatic diseases is leading in an objective assessment of the initial clinical situation, the results of treatment, the construction of further tactics of therapy and the prognosis of the disease.
Building a treatment and diagnostic plan for a patient is carried out in accordance with the diagnosis according the ICD (International Classification of Diseases), which does not reflect the pathogenesis and understanding of the driving forces of the disease.
At the end of the last century attempts were made to single out an independent discipline – clinical pathology, as a universal one for various clinical disciplines, from therapeutic to surgical, where attention is not paid to specific diseases but to their internal mechanisms. They were not a success, because they did not correspond to modern organizational and economic principles of rendering medical help.
In fact, the work of a rheumatologist includes the treatment of various forms and manifestations of inflammation. And rheumatic diseases are inflammation of the connective tissue as a whole.
Inflammation is a typical pathological process that occurs under the influence of various factors (exogenous, endogenous), characterized by a standard complex of vascular-tissue changes, regardless of the localization process and the nature of the etiological factor. The connection of the inflammatory focus with the patient’s body is established through multiple leukocyte mediators of inflammation. Inflammatory mediators induce protein synthesis in the liver, which is called the “acute phase proteins” (BOP). The first of the BOPs was C-reactive protein (CRP), then serum amyloid protein A (SAP-A), α1-antitrypsin, haptoglobulin. Active neutrophils produce inflammatory cytokines IL-1, TNF, IL-6, IL-17.
Theoretical materials on the nature of inflammation are of great practical importance. A rheumatologic patient is comorbid and in the real life various medical situations happen to him: colds, bronchitis, pneumonia, kidney and other infections. And for the choice of treatment tactics, is it important to decide whether it is an activity of a rheumatic disease or an intercurrent infection? The current state of affairs requires a radical improvement in the laboratory diagnosis of inflammation. The available methods of laboratory diagnosis do not always adequately reflect the situation in the inflammatory focus.
Traditionally, the activity of inflammation is estimated by counting the number of leukocytes and leukocyte formula (neutrophilia with a shift to the left) are calculated. But the results of the counting of leukocytes can be confusing to the doctor. In the focus of inflammation the processes of destruction may take place, and the number of leukocytes in the blood does not increase, or even decreases. In this case, leukopenia can be true and false. This is especially revealed in septic conditions due to intoxication or leukocyte adhesion to the walls of postcapillary venules.
The level of ESR also does not fully reflect the state of inflammation, as it depends on the number and properties of red blood cells. But because of its simplicity, the test continues to be widely used even in modern clinics. By its simplicity, the definition of ESR cannot be compared with any other laboratory method. It should be noted that ESR cannot serve to assess the effectiveness of treatment of the inflammatory process due to the fact that the rate of ESR after an increase can remain high for a long time (trace response).
The way out is seen in the complex definition of proteins of the acute phase of inflammation, which must be correlated with clinical and other laboratory parameters. Manifestations of the acute-phase response are also fever, leukocytosis, hyperproduction of glucocorticoids and catecholamines (adaptive hormones). At the same time, part of the proteins of the acute phase do not increase in the blood during inflammation, but on the contrary decrease due to withdrawal into the extracellular space. They are also called negative BOP – albumin (negative nitrogen balance), α and β-lipoproteins (lipolysis), transferring.
In addition, protein synthesis in the liver is reduced in cases of severe inflammation with intoxication and damage to the liver. This leads to the fact that the BOP level will be low and will not correspond to the degree of inflammation. In order to circumvent the diagnostic “trap”, it is necessary to determine not only the BOP in the blood, but also the cytokines that trigger the formation of the BOP in the liver.
In the laboratory, diagnosticums are available for various BOPs: C1-2-3-4-5, kallikrein, kininogen, plasminogen, CRP, ceruloplasmin, acid glycoprotein, antitrepsin, macroglobulin, haptoglobin, serum amyloid protein. BOPs are formed in the liver under the influence of cytokines of inflammatory cells. Adaptive hormones (GK and KA) are also involved in the induction of BOP synthesis. In most cases, hormones are prepared, and cytokines trigger the BOP synthesis. But macroglobulins are formed only under the influence of hormones. The sensitivity of the BOP results is different. The most sensitive BOP is serum amyloid protenin, then CRP, chymotrypsin, ceruloplasmin, acid glycoprotein (orosomukoid). The results of the determination of antitrypsin for the diagnosis of inflammation are not informative due to the frequent congenital deficiency of this protein. The results of the determination of haptoglobin or fibrinogen are uninformative, because they are consumed during coagulation, hemolysis, or removed from recycling through the RES. False positive results of sensitive BOP are also possible. So, SAP-A is included as a protein component in high-density lipoproteins. Therefore, its titers will depend on the lipid composition of the blood. SRB is the most popular of all BOP, especially when it is defined in the dynamics of the process. For a single determination, α1-antichymotrypsin is convenient due to its narrow norm limit and the ability to determine it by different methods.
Order of the Ministry of Health of the Russian Federation (2000) approved of the nomenclature of clinical laboratory studies on the proteins of the acute phase in blood serum, which includes:
4.1.10. Acute phase proteins in serum:
4.1.10.1. – C-reactive protein (CRP)
4.1.10.2. – serum amyloid A-protein
4.1.10.3. – acidic alpha-1-glycoprotein (orosomukoid)
4.1.10.4. – alpha-1-antitrypsin – ceruloplasmin
4.1.10.6. – haptoglobin
4.1.10.7. – fibrinogen in blood plasma
4.1.10.8. – proteins of the complement system
4.1.10.9. – prealbumin.
Diagnostic capabilities, indications for use, performance standards, interpretation of results and clinical recommendations are described in the Summary table for the practical application of laboratory diagnosis of proteins of the acute phase of inflammation at: http://visualrheumatology.ru/lab-pat-diag-vosp-rz -2.html
The use of a promising laboratory marker of inflammation in rheumatic diseases, CALPROTECTIN (CP), Calprotectin, is currently under discussion.
CP is considered as a potential acute phase marker for many inflammatory and autoimmune diseases. Calprotectin was identified in 1980 and then received the name of L-protein.
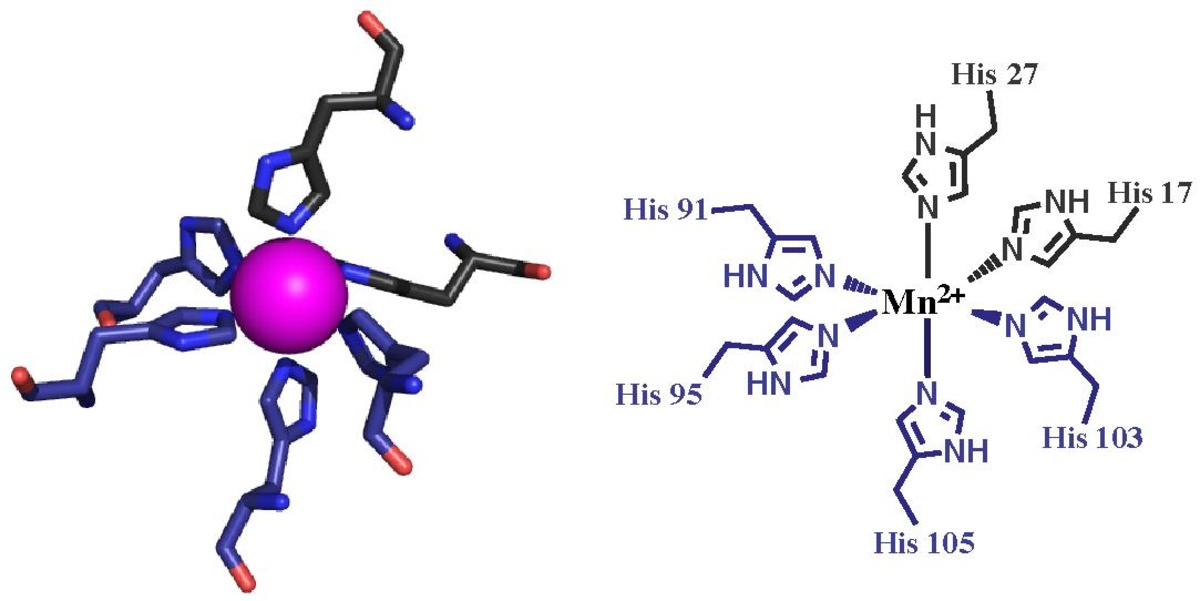 Fig. The crystal structure of calprotectin. S100A8 color gray, S100A9 purple
Gastroenterologists widely use the definition of fecal CP (PCF) currently. Analysis of calprotectin can be prescribed for symptoms such as, irregular loose stools, constipation, weight loss, fever, weakness, fatigue, impaired intestinal motility, abdominal pain, detection of blood or mucous secretions in fecal masses, dyspeptic syndrome ( nausea, vomiting), the delay of children’s developmental. Advantages of using calprotectin as a marker of inflammatory bowel disease are high sensitivity and specificity in the diagnosis of Crohn’s disease, irritable bowel syndrome. The results of the analysis are reliable and easily reproduced, since calprotectin is stable in feces for several days and is not affected by proteolytic enzymes. The method does not apply to invasive and easy to implement. This makes CP to be a universal analysis in diagnosing various pathologies in the gastrointestinal tract (inflammatory bowel pathologies; viral infections — noroviruses, adenoviruses, rotaviruses; neoplasm development, diverticulosis; reaction to various foods containing gluten). There are reference values of CP which are available for different age groups.
CP is secreted not only in the mucous membrane of the gastrointestinal tract, but also in the joints during their inflammation.
CP is a more sensitive biomarker of rheumatologic disease (RH) activity than ESR and CRP, as it directly reflects inflammation in the synovial membrane.
Data on CP, its results in RH and their clinical interpretation are presented in the Table.
Table. Practical application of laboratory diagnosis of calprotectin as a protein of the acute phase of inflammation in rheumatic diseases
Fig. The crystal structure of calprotectin. S100A8 color gray, S100A9 purple
Gastroenterologists widely use the definition of fecal CP (PCF) currently. Analysis of calprotectin can be prescribed for symptoms such as, irregular loose stools, constipation, weight loss, fever, weakness, fatigue, impaired intestinal motility, abdominal pain, detection of blood or mucous secretions in fecal masses, dyspeptic syndrome ( nausea, vomiting), the delay of children’s developmental. Advantages of using calprotectin as a marker of inflammatory bowel disease are high sensitivity and specificity in the diagnosis of Crohn’s disease, irritable bowel syndrome. The results of the analysis are reliable and easily reproduced, since calprotectin is stable in feces for several days and is not affected by proteolytic enzymes. The method does not apply to invasive and easy to implement. This makes CP to be a universal analysis in diagnosing various pathologies in the gastrointestinal tract (inflammatory bowel pathologies; viral infections — noroviruses, adenoviruses, rotaviruses; neoplasm development, diverticulosis; reaction to various foods containing gluten). There are reference values of CP which are available for different age groups.
CP is secreted not only in the mucous membrane of the gastrointestinal tract, but also in the joints during their inflammation.
CP is a more sensitive biomarker of rheumatologic disease (RH) activity than ESR and CRP, as it directly reflects inflammation in the synovial membrane.
Data on CP, its results in RH and their clinical interpretation are presented in the Table.
Table. Practical application of laboratory diagnosis of calprotectin as a protein of the acute phase of inflammation in rheumatic diseases
Determining the concentration of calprotectin in the serum of patients is possible by ELISA using test systems Immun diagnostik (Germany).
CONCLUSIONS: Studying the level of CP can be useful for more accurate assessment of disease activity, identifying “residual” inflammation and predicting the exacerbation of the pathological process when planning a strategy for further therapy. CP is a promising marker in monitoring the effectiveness of therapy for DMARDs and GIBP, as well as for predicting its results. Elevated levels of CP may be a marker for the development of erosive joint damage in RA. The value of CP in SpA is ambiguous: on the one hand, this marker is highly expressed in the synovial tissue of patients with SpA and correlates with the level of acute phase indicators, on the other hand – its correlation with disease activity indices is very controversial and requires further clarification. Serum and fecal CP can serve as a useful marker for the detection of subclinical inflammation of the intestinal wall of patients with SpA, which must be considered when treating this group of patients. (Scientific and Practical Rheumatology. 2018; 56 (4): 494-499.)
 Fig. The crystal structure of calprotectin. S100A8 color gray, S100A9 purple
Gastroenterologists widely use the definition of fecal CP (PCF) currently. Analysis of calprotectin can be prescribed for symptoms such as, irregular loose stools, constipation, weight loss, fever, weakness, fatigue, impaired intestinal motility, abdominal pain, detection of blood or mucous secretions in fecal masses, dyspeptic syndrome ( nausea, vomiting), the delay of children’s developmental. Advantages of using calprotectin as a marker of inflammatory bowel disease are high sensitivity and specificity in the diagnosis of Crohn’s disease, irritable bowel syndrome. The results of the analysis are reliable and easily reproduced, since calprotectin is stable in feces for several days and is not affected by proteolytic enzymes. The method does not apply to invasive and easy to implement. This makes CP to be a universal analysis in diagnosing various pathologies in the gastrointestinal tract (inflammatory bowel pathologies; viral infections — noroviruses, adenoviruses, rotaviruses; neoplasm development, diverticulosis; reaction to various foods containing gluten). There are reference values of CP which are available for different age groups.
CP is secreted not only in the mucous membrane of the gastrointestinal tract, but also in the joints during their inflammation.
CP is a more sensitive biomarker of rheumatologic disease (RH) activity than ESR and CRP, as it directly reflects inflammation in the synovial membrane.
Data on CP, its results in RH and their clinical interpretation are presented in the Table.
Table. Practical application of laboratory diagnosis of calprotectin as a protein of the acute phase of inflammation in rheumatic diseases
Fig. The crystal structure of calprotectin. S100A8 color gray, S100A9 purple
Gastroenterologists widely use the definition of fecal CP (PCF) currently. Analysis of calprotectin can be prescribed for symptoms such as, irregular loose stools, constipation, weight loss, fever, weakness, fatigue, impaired intestinal motility, abdominal pain, detection of blood or mucous secretions in fecal masses, dyspeptic syndrome ( nausea, vomiting), the delay of children’s developmental. Advantages of using calprotectin as a marker of inflammatory bowel disease are high sensitivity and specificity in the diagnosis of Crohn’s disease, irritable bowel syndrome. The results of the analysis are reliable and easily reproduced, since calprotectin is stable in feces for several days and is not affected by proteolytic enzymes. The method does not apply to invasive and easy to implement. This makes CP to be a universal analysis in diagnosing various pathologies in the gastrointestinal tract (inflammatory bowel pathologies; viral infections — noroviruses, adenoviruses, rotaviruses; neoplasm development, diverticulosis; reaction to various foods containing gluten). There are reference values of CP which are available for different age groups.
CP is secreted not only in the mucous membrane of the gastrointestinal tract, but also in the joints during their inflammation.
CP is a more sensitive biomarker of rheumatologic disease (RH) activity than ESR and CRP, as it directly reflects inflammation in the synovial membrane.
Data on CP, its results in RH and their clinical interpretation are presented in the Table.
Table. Practical application of laboratory diagnosis of calprotectin as a protein of the acute phase of inflammation in rheumatic diseases
| KALPROTEKTIN (CP) Calprotectin | Note | |
| PROTEIN TYPE | Calprotectin (CP) belongs to the S100 family of leukocyte proteins. This is a non-covalent heterodimer with a molecular mass of 36.5 kDa, consisting of two protein calcium-binding molecules – S100A8 and S100A9 (MRP8 / 14, calgranulin A / B) encoded by a gene located on chromosome 1q21. CP contains zinc-binding domains, so it has antimicrobial activity. MRP8 / 14 – the main intracellular protein of neutrophil granulocytes and monocytes, in the cytosol of which its content is 40–60% of the total amount of proteins; it is practically absent in the lymphocyte cytoplasm. CP is a heinogenic toll-like receptor 4 ligand (TLR4); It has a pro-inflammatory effect on phagocytes and endothelial cells in vitro and contributes to the development of the inflammatory process in vivo; It is an important mediator of many regulatory functions, such as chemotaxis, activation of neutrophil degranulation and phagocytosis, inhibition of the synthesis of immunoglobulins, cell proliferation and differentiation | |
| Calprotectin functions | The main intracellular protein of neutrophilic granulocytes and monocytes. It has a pro-inflammatory effect on phagocytes and endothelial cells. Mediator of many regulatory functions (chemotaxis, activation of neutrophil degranulation and phagocytosis, inhibition of the synthesis of immunoglobulins, cell proliferation and differentiation. | |
| Biological effects of calprotectin | Antifungal action; inhibits the reproduction of microorganisms; contributes to the programmed death (apoptosis) of benign and malignant tumor cells due to the release of chemokines and tumor necrosis factor (TNF). | This is done through competitive bidding to zinc ions and blocking zinc-dependent enzymes necessary for the functioning of microorganisms. |
| Calprotectin with RA | ||
| Indications for RA | monitoring of RA activity, detection of subclinical inflammation and prediction of exacerbations of the disease. evaluation of disease activity in the early stages of RA. monitoring the effectiveness of therapy with various genetically engineered biological preparations (GIBP) and predicting its results. CP level monitoring to detect “residual” inflammation in patients in remission / low disease activity | A reliable relationship was established between the level of CP and ESR, the concentration of CRP, as well as clinical and ultrasound (US) indicators of the activity of inflammation. A CP level> 1.66 mg / ml is associated with the presence of an active synovitis according to ultrasound data: using CRP or ESR to detect synovitis gives a less significant result. |
| The multiplicity of the survey | initially and after 6, 12 months. | Independent predictor of radiological progression of destructive changes in the joints |
| CP in the treatment of RA RTM | Control after 24 weeks | The division into “answered” and “not answered.” The only independent predictor of the effectiveness of RTM therapy |
| CP in the treatment of RA etanercept (ETC). | Control after 12.25 weeks | significant correlation between the concentration of CP and ESR, the level of CRP, NPV, index DAS28. |
| CP in the treatment of MT juvenile RA (JRA) | CP when predicting the effectiveness of therapy with methotrexate (MT) | Associated with the achievement of ACR50 in contrast to other parameters (CRP, ESR levels IL2, IL6, IL12, IL18 and TNFα). |
| Calprotectin for spondyloarthritis | ||
| Calprotectin for spondyloarthritis | CP level was significantly increased in the synovial fluid of patients with SpA, and its positive correlation with acute phase indicators was also recorded. | The literature data on the clinical significance of KP in spondyloarthritis (SpA) are very contradictory. |
| SpA and intestinal lesions | about half of patients with SpA have subclinical inflammation of the intestine, which can lead to an increase in serum KP concentration, which can also distort the picture of laboratory inflammation | Diagnosis of inflammation of the intestinal mucosa requires additional instrumental methods |
| algorithm for the diagnosis of subclinical inflammation of the intestine with SpA | initial assessment of serum CP and CRP and subsequently, if necessary, assessment of the level of fecal CP | the use of this algorithm allows to detect inflammation of the intestine with a probability of 74.4%. |
| CP during therapy with TNFα inhibitors with axial and peripheral SpA | monitoring of disease activity during therapy, together with the level of CRP predictor of radiological progression of spinal lesions in axial spA | Monitoring Level CP is rapidly reduced during therapy with TNFα inhibitors A concentration of CP> 0.5 mg / ml was associated with the progression of the growth of syndesmophytes during a two-year observation of KP. It can be considered as an acute-phase indicator, the level of which reflects more accurately the inflammation of the synovial membrane of the joints, compared with CRP and ESR. |
| CP at other RZ | ||
| CP and other RP | stille disease Sjogren’s syndrome, systemic lupus erythematosus osteoarthritis | Promising laboratory marker for monitoring disease activity. The level of KP is the highest among patients with Still’s disease and reliably correlates with the content of ferritin and inversely correlates with the level of hemoglobin. |


